(PRESS RELEASE) VANCOUVER, Canada—True Leaf Medicine Inc. (True Leaf), a division of True Leaf Medicine International Ltd. (MJ:CSE) (TLA:FSE) (TRLFF:OTCQB), is in the final stage of the process to acquire a license to produce marijuana in Canada.
Today, the company responded to a detailed request for information about its planned operation in Lumby, B.C. from Health Canada.
The request came as part of the Detailed Review and Security Clearance stage of Health Canada’s Access to Cannabis for Medical Purposes Regulations (ACMPR). Once True Leaf successfully completes this final stage, the company can expect to be granted a license to cultivate cannabis and produce marijuana in Canada. Health Canada will then schedule an inspection to verify that True Leaf is meeting the requirements of the ACMPR, and if the company successfully completes that step, Health Canada will issue a license for True Leaf to sell cannabis products in Canada.
The ACMPR process is preparing Canada for the passage into law of the Cannabis Act, governing the legalization, regulation and control of marijuana, which is expected in 2018. True Leaf is working to ensure the company will comply with the regulations directed by Health Canada under this new legislation, to allow the company to participate in all aspects of marijuana production granted to licensed producers.
True Leaf CEO Darcy Bomford has shepherded the True Leaf application from the first day it was submitted to Health Canada on July 19, 2013.
“It’s been a long process,” he said, “but True Leaf’s team fully understands the importance of getting it right and ensuring that security and quality are paramount. We’re believers in the healing potential of cannabis and we’re committed to improving the quality of our customers’ lives. So we fully support Health Canada’s efforts to ensure a secure, quality supply.”
True Leaf’s latest submission responds to detailed questions from Health Canada about the size, capacity, and security measures on site and future expansion plans. True Leaf is working with industry experts to ensure the company meets or exceeds the operating, facility, security and reporting procedures during this step in the ACMPR application process.
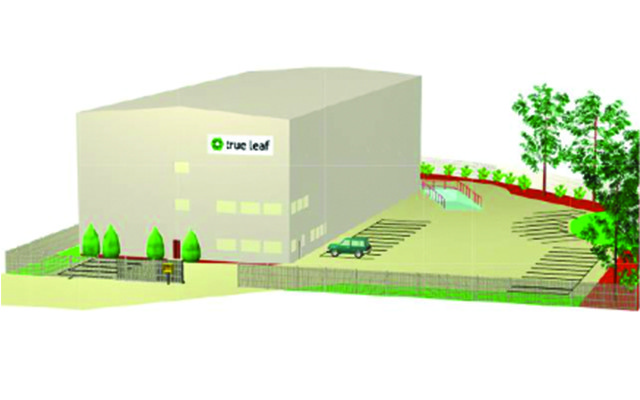
Currently, the plan for the first phase calls for a 48,000-square-foot facility that will meet ACMPR requirements. As demand for inventory grows, True Leaf will expand its operation to meet the market demand based on its license-to-produce allocation.
The facility’s initial production is anticipated to begin in April 2018 with a target of 5,200 kilograms of cannabis per annum.
True Leaf is one of 428 companies that have applications in the Detailed Review stage for a license to produce marijuana, out of a total of 1,665 applications received by Health Canada under the Access to Cannabis for Medical Purposes Regulations (ACMPR). Today, there are just 52 licensed producers in Canada.
As True Leaf goes through the ACMPR licensing process, the company has increased value for shareholders by creating its pet division, True Leaf Pet. The company has been marketing hemp-based supplements for pets since 2015, which are now distributed throughout North America and Europe. True Leaf’s work in the pet industry is valuable experience that can be leveraged to build out the company’s future health and wellness line of high-CBD cannabis products for humans when it becomes a licensed producer.